Research
Stress responses of neuronal cells
Background
One of our works aimed at deciphering the underlying mechanisms how neuronal cells monitor and respond to various stressors. We used two cell lines, Ht22 murine hippocampal neuronal cell line and PC12 rat adrenal pheochromocytoma cell line, for the investigation.
Neuroprotective mechanisms activated by different intensity of stress in HT22 cells
 Fig.1 HT22 murine hippocampal neuronal cell
HT22 murine hippocampal neuronal cell does not possess glutamic receptor, thereby often used for analyzing how neuronal cells respond against oxidative stress (Fig. 1). We stimulated HT22 cells with various concentration of glutamic acid and/or H2O2, and investigated cellular responses. As expected, treating cells with high concentration of glutamic acid or H2O2 resulted in induction of cell death. Intriguingly, on the contrary, treating cells with very low concentration of these compounds induced cell survival.
Fig.1 HT22 murine hippocampal neuronal cell
HT22 murine hippocampal neuronal cell does not possess glutamic receptor, thereby often used for analyzing how neuronal cells respond against oxidative stress (Fig. 1). We stimulated HT22 cells with various concentration of glutamic acid and/or H2O2, and investigated cellular responses. As expected, treating cells with high concentration of glutamic acid or H2O2 resulted in induction of cell death. Intriguingly, on the contrary, treating cells with very low concentration of these compounds induced cell survival.
To elucidate the molecular mechanisms underlying this low concentration of glutamic acid and/or H2O2 induced cell survival, we are now focusing on MAP kinase family (Erk1/2, JNK. p38, etc.) which is reportedly activated by oxidative stress. Our preliminary data suggested that the low intensity of oxidative stress only activated Erk1/2, therefore, we hypothesizing that the different sensitivity of MAP kinase family activation by oxidative stress may determine cellular fates.
As we mentioned below, a high intensity of oxidative stress induces HT22 cell death; however, even at this conditions, HT22 cells appeared to activate neuroprotective mechanisms via growth factors which acts in autocrine/paracrine fashion. We are also investigating the molecular mechanisms underlying this neuroprotection.
Mechanisms of neuroprotection by oxidative preconditioning
Oxidative stress is generated by increasing in reactive oxygen species (ROS) produced from mitochondria, thereby often inducing toxic effect on cell functions. Recently, it has been demonstrated that administration of low concentration H2O2, possibly generates a weak oxidative stress, sometimes increased oxidative stress tolerance. Several reports suggested increasing in antioxidant proteins, such as catalase and super oxide dismutase (SOD), contributed in this stress tolerance; however, the detail of this mechanism is still not fully understood, and it is also unclear w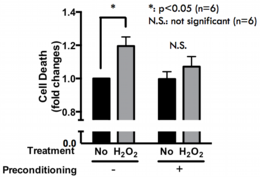 Fig.2 Effects of oxidative preconditioning in PC12 cellshether the other mechanisms are involved in this process. To elucidate these questions, we established oxidative stress tolerance model using PC12 cells. Briefly, the administration of low concentration of H2O2 (1~10 μM) to PC12 cells for 24 hours (pretreatment) abolished high concentration H2O2 (100~250 μM) induced celldeath (Fig. 2). By using this model, we are now studying how this stress tolerance was occurred.
Fig.2 Effects of oxidative preconditioning in PC12 cellshether the other mechanisms are involved in this process. To elucidate these questions, we established oxidative stress tolerance model using PC12 cells. Briefly, the administration of low concentration of H2O2 (1~10 μM) to PC12 cells for 24 hours (pretreatment) abolished high concentration H2O2 (100~250 μM) induced celldeath (Fig. 2). By using this model, we are now studying how this stress tolerance was occurred.
Role of growth factor progranulin in brain
PGRN in NPCs
 Fig.3 NPCs obtained from murine hippocampusProgranulin (PGRN) is a growth factor thought to affect multiple processes in the central nerves system (CNS), including brain sexual differentiation, adult neurogenesis, and development of neurodegenerative diseases. We have previously demonstrated that PGRN stimulates neural progenitor cell (NPC) proliferation via GSK3beta inactivation. On the other hand, the detail PGRN signaling in NPCs remains elusive. We recently utilized microarray analysis to compare the gene expression profiles between WT- and PGRN KO-NPCs, and identified several genes related to neuroregeneration, neuroprotection, and cell cycle that were downregulated in KO-NPCs. We are now poised to determine the detail roles of these gene expressional changes in KO-NPCs.
Fig.3 NPCs obtained from murine hippocampusProgranulin (PGRN) is a growth factor thought to affect multiple processes in the central nerves system (CNS), including brain sexual differentiation, adult neurogenesis, and development of neurodegenerative diseases. We have previously demonstrated that PGRN stimulates neural progenitor cell (NPC) proliferation via GSK3beta inactivation. On the other hand, the detail PGRN signaling in NPCs remains elusive. We recently utilized microarray analysis to compare the gene expression profiles between WT- and PGRN KO-NPCs, and identified several genes related to neuroregeneration, neuroprotection, and cell cycle that were downregulated in KO-NPCs. We are now poised to determine the detail roles of these gene expressional changes in KO-NPCs.
On the other hand, intriguingly, we found that PGRN KO-NPCs were more vulnerable compared to WT-NPCs; however, this vulnerability was not reversed by the addition of exogenous PGRN - which may indicate “intracellular” PGRN is important -, therefore, we are now exploring the other methods to rescue the phenotype of KO-NPCs.
Besides, we also focus on the expressional control of PGRN in neuronal cells.
Nutrients and neuronal cells
SIRT1 and FOXOs in neuronal cells
Nutrient availability is one of the most important signals that regulate cellular fates including cell growth, differentiation, and death. Accumulated evidence suggested NAD+-dependent histone deacethylase, sirtuin 1 (SIRT1) plays prominent role on connecting between changes in nutritional availability and regulation of cellular fates. More recently, one of the forkhead box O (FOXO) family, FOXO3, was identified to mediate, at least in a part, SIRT1 action. The expression of SIRT1 and FOXO3 is observed in the nerve cells, whereas, the expressional and functional regulation of these proteins is not fully understood.
Thus, the aim of the study is that to elucidate whether the glucose availability affects the functions of SIRT1 and FOXO using PC12 cells. Total expression level of SIRT1 and FOXO in PC12 cells was reduced in response to increasing in glucose availability via gene expression control. Intracellular localization of SIRT1 and FOXO was also affected by glucose availability. 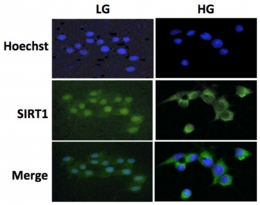 Fig.4 Intracellular localization of SIRT1 in response to changes in glucose availabilityAlthough the glucose-dependent regulation of SIRT1 and FOXO was very similar, the influence of NGF on these proteins was completely differed. SIRT1 amounts were increased by NGF treatment, whereas, FOXO amounts were rather reduced by NGF treatment in a gene expression-independent manner.
Fig.4 Intracellular localization of SIRT1 in response to changes in glucose availabilityAlthough the glucose-dependent regulation of SIRT1 and FOXO was very similar, the influence of NGF on these proteins was completely differed. SIRT1 amounts were increased by NGF treatment, whereas, FOXO amounts were rather reduced by NGF treatment in a gene expression-independent manner.
Overall, behaviors of SIRT1/FOXO were synchronously regulated by changes in glucose availability in PC12 cells; whereas, NGF treatment differentially controlled SIRT1 and FOXO which may be an important mechanism that determine the neuronal cell fate.
Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle
It has been well documented that an appropriate amount of physical exercise exerts a wide variety of biological effects, such as an enhancement of glucose disposal, changes in muscle fiber types, induction of angiogenesis, and regulation of the immune system. In addition, adequate exercise is highly beneficial not only for preventing the development of type 2 diabetes but also for diabetic patients already suffering from insulin resistance. However, the molecular basis of these beneficial effects of exercise/skeletal muscle contraction is still poorly understood, and elucidation of the mechanisms by which skeletal muscle cells decipher and respond to contractile stimuli is a major issue in understanding the beneficial and in some cases detrimental effects of exercise.
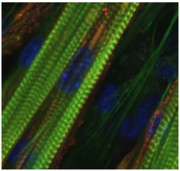 Fig.5 C2C12 myotubes suitable for in vitro contraction systemWe recently established a novel skeletal muscle contraction model based on the use of contractile C2C12 myotubes to explore contraction-inducible changes in contracting muscle cells. By using this advanced in vitro contraction system, we succeeded in identifying mouse CXCL1/KC and CXCL5/LIX as novel candidates for exercise factors that may contribute to mediating the effects of exercise. We are continuously exploring novel exercise-dependent bio-actions by using this model.
Fig.5 C2C12 myotubes suitable for in vitro contraction systemWe recently established a novel skeletal muscle contraction model based on the use of contractile C2C12 myotubes to explore contraction-inducible changes in contracting muscle cells. By using this advanced in vitro contraction system, we succeeded in identifying mouse CXCL1/KC and CXCL5/LIX as novel candidates for exercise factors that may contribute to mediating the effects of exercise. We are continuously exploring novel exercise-dependent bio-actions by using this model.
